From agriculture to textiles, leather manufacturing, and chemical synthesis, organic acids are vital raw materials for many industrial processes.
Among these, formic acid (HCOOH) and acetic acid (CH₃COOH) are two of the most widely used. While both are simple carboxylic acids, their chemical properties, production methods, industrial applications, safety profiles, and environmental impacts vary significantly.
In this article, we analyze the differences between formic acid and acetic acid from a professional supplier’s standpoint, helping industry users make informed choices based on their application needs.
Chemical Structure and Physical Properties
The most basic carboxylic acid is formic acid, which is more acidic than acetic acid (lower pKa). In some chemical reactions, this makes it a better reducing agent and more reactive.
| Property | Formic Acid (HCOOH) | Acetic Acid (CH₃COOH) |
| Molecular Formula | HCOOH | CH₃COOH |
| Molar Mass (g/mol) | 46.03 | 60.05 |
| Density (g/cm³) | 1.22 at 20°C | 1.05 at 20°C |
| Boiling Point (°C) | 100.8 | 118.1 |
| Melting Point (°C) | 8.4 | 16.6 |
| pKa (acid dissociation) | 3.75 | 4.76 |
| Odor | Pungent, irritating | Vinegar-like |
| Solubility in water | Miscible | Miscible |
Methods of Production
Formic Acid
Formic acid is produced mainly by:
Methanol reacts with carbon monoxide to form methyl formate, then hydrolyzed using sodium methoxide.
Oxidation of biomass or formaldehyde for sustainable routes.
Industrial Reaction:
CH₃OH + CO → HCOOCH₃ → HCOOH + CH₃OH
Acetic Acid
Produced through:
- Methanol carbonylation (Monsanto and Cativa processes), the most dominant industrial method.
- Oxidative fermentation of ethanol (used in food-grade vinegar production).
- Oxidation of acetaldehyde or butane (less common).
Industrial Reaction:
CH₃OH + CO → CH₃COOH
Comparison
Both acids are derived from carbon monoxide and methanol in synthetic processes. However, formic acid production is slightly less efficient and costlier at large scale due to lower demand and specialized handling needs.
Industrial Applications
Formic acid is more often used where acidity and reactivity are required in smaller quantities (e.g., animal feed, coagulants). Acetic acid is widely used in large volumes, mainly for producing various industrial chemicals.
| Application Area | Formic Acid | Acetic Acid |
| Leather & Textile | Deliming, dyeing | Dye fixing, desizing |
| Rubber Industry | Coagulation of latex | Plasticizer synthesis |
| Agriculture | Preservative for silage | Herbicide component |
| Food Industry | Antibacterial additive (E236) | Vinegar, preservative (E260) |
| Chemical Intermediate | Pharmaceuticals, pesticides, esters | Vinyl acetate, acetic anhydride, esters |
| Cleaning Products | Limescale remover | Descaler, disinfectant |
| Oil & Gas Industry | Acidizing agent in oil well stimulation | pH control in drilling |
| Laboratory Use | Reducing agent, analytical reagent | Solvent, buffer component |
Reactivity and Chemical Behavior
Acid Strength
Formic acid (pKa ≈ 3.75) is a stronger acid than acetic acid (pKa ≈ 4.76). Therefore, in reactions where stronger acid catalysis or faster proton donation is required, formic acid is more efficient.
Reducing Properties
Formic acid is a known reducing agent. It decomposes into CO and H₂O under heat or in the presence of catalysts, making it useful in reduction reactions and as a hydrogen source in fuel cells.
Acetic acid, while reactive, does not exhibit strong reducing properties and is typically more stable under high temperatures.
Hydrogen Bonding
Both acids form hydrogen bonds, but formic acid forms stronger ones due to its smaller molecular size. This affects solubility and volatility.
Safety and Handling
Formic acid demands more stringent safety measures because of its strong corrosiveness and toxic fumes. Acetic acid, though pungent and flammable, is generally safer to handle, especially when diluted.
| Parameter | Formic Acid | Acetic Acid |
| Corrosiveness | Highly corrosive, especially vapor | Irritant, but less corrosive |
| TLV (Threshold Limit Value) | 5 ppm | 10 ppm |
| Flash Point | 69°C | 40°C |
| Inhalation Risk | Can cause respiratory distress | Irritating at high concentrations |
| Skin Contact | Causes burns, blisters | Can irritate skin or cause rash |
Environmental Impact and Biodegradability
Both formic and acetic acids are readily biodegradable in the environment.
Formic acid is less likely to build up in ecosystems since it naturally breaks down into carbon dioxide and water. It is considered eco-friendly in agriculture as a feed preservative.
Acetic acid, especially in its diluted form, poses minimal ecological threat and is used in eco-cleaning agents.
Important Note
In concentrated form, both acids can cause localized environmental damage to aquatic life if spilled or improperly discharged.
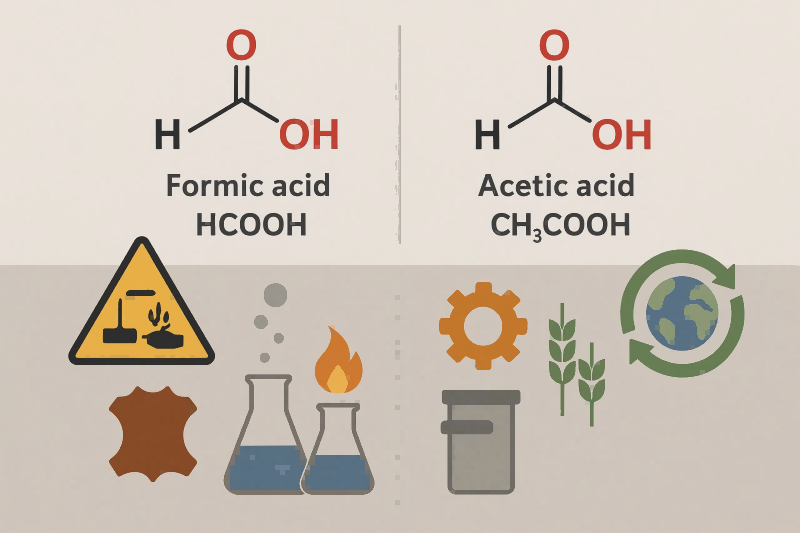
Economic Factors and Market Demand
Global Market Price (as of 2024)
| Chemical | Price (USD/ton, average) |
| Formic Acid | $700–$950 |
| Acetic Acid | $450–$600 |
Production Volume
Acetic acid enjoys significantly higher global production (~16 million tons/year) due to its wider industrial application.
Formic acid production is much lower (~1 million tons/year), making it a more niche chemical.
Market Trends
Acetic acid sees steady demand in plastic production (e.g., polyvinyl acetate), adhesives, and solvents.
Formic acid demand is growing in eco-agriculture and feed additives due to antimicrobial properties and EU regulations limiting antibiotics in livestock.
Regulatory Status
| Region | Formic Acid | Acetic Acid |
| EU | Approved as E236 (preservative) | Approved as E260 (acidifier, preservative) |
| USA (FDA) | limited in food; GRAS in animal feed | GRAS (Generally Recognized As Safe) |
| China | Widely used in rubber & leather | Industrial and food use allowed |
| REACH | Registered and restricted | Fully registered under REACH |
Both acids are generally safe under regulatory use conditions but formic acid’s corrosiveness limits its direct food applications.
How to Choose Between Formic Acid and Acetic Acid?
Choose Formic Acid
Opt for formic acid when you need a stronger, more reactive acid in smaller quantities. Its higher acidity and reducing properties make it ideal for use in latex coagulation, leather processing, animal feed preservation, and certain organic synthesis reactions. It’s especially useful in applications that demand strong antimicrobial effects or require controlled pH in concentrated environments. However, because of its corrosive nature, it needs to be handled carefully while wearing the appropriate safety gear.
Choose Acetic Acid
Select acetic acid for large-scale applications where cost-efficiency, mild acidity, and safer handling are priorities. It is the better choice for producing plastic precursors, solvents, vinegar, and cleaning agents. Acetic acid is widely used in the textile, food, chemical, and adhesive industries, thanks to its milder nature, availability in bulk, and low cost. It’s also the preferred acid in settings requiring food-grade or household-safe ingredients.
Consider the Industry and End Use
Your choice depends heavily on what you’re producing. For industrial chemicals and large-volume synthesis, acetic acid is the standard. For specialized or reactive applications, especially in agriculture, leather, or rubber processing, formic acid may be more effective.
Think About Safety and Storage
Formic acid requires stricter handling due to its higher corrosiveness and lower safety threshold. If the working environment prioritizes ease of storage, transportation, or operator safety, acetic acid is the more practical choice.
Evaluate Environmental and Regulatory Needs
Both acids are biodegradable, but if you’re targeting eco-friendly animal feed or sustainable preservatives, formic acid may offer better antimicrobial performance under regulatory limits.