Calcium hypochlorite (Ca(ClO)₂) and sodium hypochlorite (NaClO) are two of the most commonly used chlorine-based disinfectants across municipal, industrial, and commercial sectors. Both release hypochlorous acid (HOCl), the primary antimicrobial agent responsible for oxidizing and deactivating pathogens. However, their differences in chemical composition, handling characteristics, storage requirements, and economic considerations play a crucial role in industrial selection.
Chemical Properties Overview
Calcium Hypochlorite (Ca(ClO)₂)
- Formula: Ca(ClO)₂
- Appearance: White or grayish-white solid, usually in granular or tablet form
- Available chlorine: 65–70%
- Stability: More stable in solid form
- pH in solution: 10–12 (alkaline)
- Solubility in water: Moderate
Pros | Cons |
|
|
Sodium Hypochlorite (NaOCl)
- Formula: NaOCl
- Appearance: Pale greenish-yellow liquid
- Available chlorine: 5–15% in commercial products
- Stability: Less stable; degrades faster over time, especially with heat and sunlight
- pH in solution: 11–13 (alkaline)
- Solubility in water: Completely soluble
Pros | Cons |
|
|
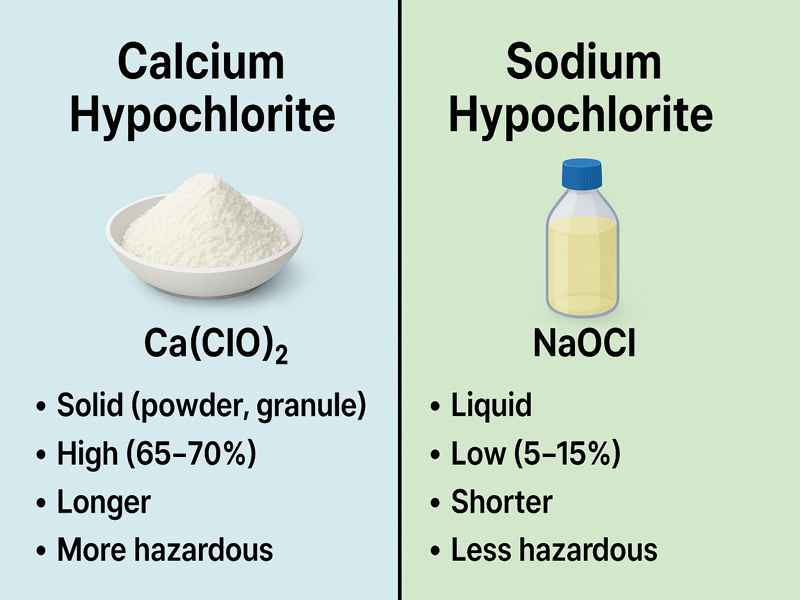
| Property | Calcium Hypochlorite | Sodium Hypochlorite |
| Physical form | Solid (powder, granule) | Liquid |
| Chlorine concentration | High (65–70%) | Low (5–15%) |
| Shelf life | Longer | Shorter |
| Transport hazard | More hazardous | Less hazardous |
Production
Calcium Hypochlorite
The usual method for producing calcium hypochlorite is a two-step reaction that includes:
Chlorine gas and calcium hydroxide (slaked lime) react as follows:
- 2Cl2+2Ca(OH)2→Ca(OCl)2+CaCl2+2H2O
- Filtration, drying, and compression into granules or tablets.
Due to its solid state and oxidative nature, dust control and moisture protection are key during packaging and storage.
Sodium Hypochlorite
Commercial NaOCl is produced via the Hooker process:
- Cl2+2NaOH→NaCl+NaOCl+H2O
- It is typically diluted to ~12.5% for industrial use or 5–6% for household bleach. It must be stored in UV-protected containers and used within weeks to avoid decomposition into chlorate and salt.
Summary: Calcium hypochlorite is more stable during shipping and long-term storage. Sodium hypochlorite, while easier to produce and transport in bulk, has higher degradation risks.
Storage and Stability
Calcium Hypochlorite
- Shelf life: Up to 12–18 months in sealed containers
- Decomposes slowly when exposed to heat and humidity
- Strong oxidizer; must not be stored near organic materials
- Tablets minimize spillage and allow for easier dose control
Sodium Hypochlorite
- Shelf life: 1–3 months for concentrated solutions
- Rapid degradation at temperatures above 25°C or in sunlight
- Requires pH stabilization (usually kept >11) to prevent decomposition
- Incompatible with acids and ammonia-containing products
Effectiveness and Usage
Disinfection Strength
- Calcium hypochlorite is stronger on a per-gram basis due to higher available chlorine content.
- Sodium hypochlorite, being a diluted liquid, requires larger volumes to match disinfection power.
Residual Chlorine
- Calcium Hypochlorite leaves more solid residues, which may need removal in some systems.
- Sodium Hypochlorite produces cleaner dissolution but may result in quicker chlorine decay.
Dosage and Management
| Feature | Calcium Hypochlorite | Sodium Hypochlorite |
| Ease of measurement | Requires careful weighing | Easily measured in liters |
| Dosing method | Dry feed or dissolved first | Pumped directly as liquid |
| Equipment needs | Dry feeder or dissolver | Chemical feed pumps |
Cost Analysis
| Cost Factor | Calcium Hypochlorite | Sodium Hypochlorite |
| Approximate Unit Price | ~$1.5–2.0/kg (solid, 65–70% available chlorine) | ~$0.3–0.5/L (12.5% available chlorine) |
| Chlorine Delivery Efficiency | High (solid, less volume needed) | Low (bulk liquid required) |
| Logistics Cost | Higher due to solid hazardous class | Lower per liter, but requires more volume |
Safety and Environmental Considerations
Safety Precautions
- Calcium Hypochlorite: Highly reactive, especially with organics. It must be stored separately and kept out of direct sunlight. Protective gloves and goggles are essential during handling.
- Sodium Hypochlorite: Corrosive and can release toxic chlorine gas if mixed with acids. Requires ventilation and eye protection.
Environmental Impact
- Neither should be dumped straight into rivers since they are harmful to aquatic life.
- Excess chlorine can form disinfection by-products (DBPs) like trihalomethanes (THMs) and haloacetic acids (HAAs), especially in municipal water treatment.
Applications
Although both substances are used for bleaching and disinfection, their precise applications are different.
Calcium Hypochlorite Applications:
- Swimming pool sanitation
- Drinking water disinfection (especially in remote or emergency situations)
- Wastewater treatment
- Bleaching in the textile and paper industries
- Emergency response (e.g., cholera outbreaks, disaster zones)
Sodium Hypochlorite Applications:
- Household bleach and cleaners
- Water treatment plants (municipal use)
- Surface disinfection in hospitals, schools, and food facilities
- Industrial cleaning (equipment, tanks, pipelines)
- Laundry and textile bleaching
Regulatory and Standardization Overview
| Regulatory Body | Calcium Hypochlorite | Sodium Hypochlorite |
| NSF/ANSI 60 | Approved for potable water disinfection | Approved |
| WHO Guidelines | Included as a recommended disinfectant | Included |
| EPA (USA) | Registered disinfectant, regulated under FIFRA | Registered |
| EU (REACH) | Classified as a hazardous substance | Also hazardous, requires labeling |
| UN Transport Code | UN2880, Class 5.1 oxidizer | UN1791, Class 8 corrosive |
Which One Should You Choose?
Choosing between calcium hypochlorite and sodium hypochlorite depends on your specific application, infrastructure, safety priorities, and cost constraints.
- If you need a highly concentrated, long-lasting, and portable disinfectant, calcium hypochlorite is ideal, especially for remote areas, emergency kits, and pool maintenance.
- If your operation requires ease of use, automated dosing, or immediate availability in liquid form, sodium hypochlorite is more suitable, particularly for municipalities, homes, and industrial systems.
Understanding these differences allows chemical buyers and users to make informed, safe, and cost-effective decisions in various sectors, from public health to industrial sanitation.