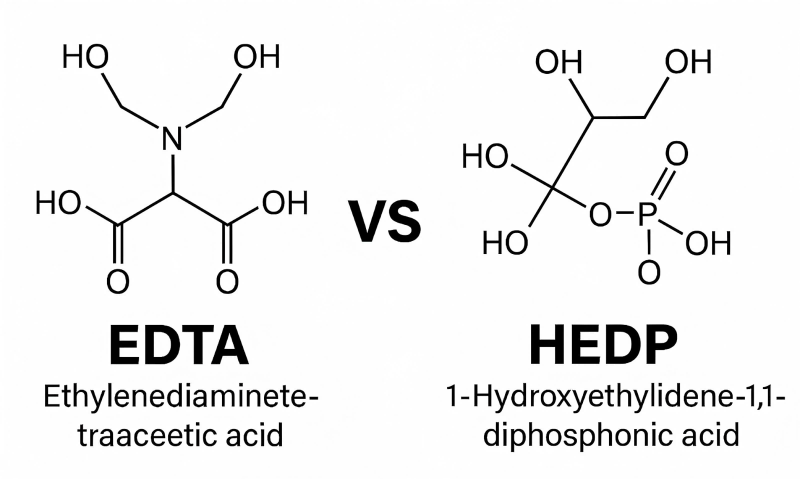
In the realm of water treatment, industrial cleaning, cosmetics, pharmaceuticals, and textile processing, chelating agents and scale inhibitors are indispensable. Two of the most widely used chemicals in these applications are EDTA (Ethylenediaminetetraacetic acid) and HEDP (1-Hydroxyethylidene-1,1-diphosphonic acid).
At first glance, both compounds serve similar functions — binding metal ions and controlling scale. However, as a professional supplier of chemical raw materials, we understand that these two chemicals differ significantly in structure, behavior, application, pH stability, and environmental impact.
This article offers a deep technical and application-based comparison between EDTA and HEDP to help industrial users, formulators, and purchasing agents make informed decisions.
Chemical Identity and Structure
EDTA (Ethylenediaminetetraacetic Acid)
- Chemical Formula: C₁₀H₁₆N₂O₈
- Molecular Weight: 292.24 g/mol (acid form)
- Structure: Contains two amine groups and four carboxylic acid groups
- CAS Number: 60-00-4
- Nature: Aminopolycarboxylic acid
- Typical Forms: EDTA acid, disodium salt (EDTA-2Na), tetrasodium salt (EDTA-4Na)
With six coordination sites, EDTA is a chelating agent. Along with many other metal ions, it forms stable complexes with Ca2+, Mg2+, Fe2+, and Cu2+.
HEDP (1-Hydroxyethylidene-1,1-diphosphonic Acid)
- Chemical Formula: C₂H₈O₇P₂
- Molecular Weight: 206.03 g/mol
- Structure: Phosphonate group with a hydroxyethylidene backbone
- CAS Number: 2809-21-4
- Nature: Organophosphonate
- Typical Forms: HEDP acid, HEDP-Na (mono, di, tetra sodium salts)
HEDP is primarily a scale inhibitor and corrosion inhibitor. It can chelate some metal ions but its primary function is to stabilize metal ions in aqueous systems and prevent precipitation.
Functional Differences
| Aspect | EDTA | HEDP |
| Primary Function | Chelating agent | Scale inhibitor and corrosion inhibitor |
| Metal Binding Sites | 6 (hexadentate ligand) | 4 (bidentate through phosphonate groups) |
| Key Applications | Cosmetics, pharmaceuticals, cleaning, textiles | Water treatment, oilfield, cooling towers, boilers |
| Preferred pH Range | Neutral to mildly alkaline (4–10) | Wide pH range; effective in acidic to alkaline environments |
| Chelating Strength (Fe³⁺) | High | Moderate |
| Scale Inhibition | Moderate | Excellent |
| Corrosion Inhibition | Low | High |
Chelation Capability
EDTA: Superior Chelator
EDTA is a strong hexadentate chelating agent, meaning it can bind to a single metal ion at six different sites. This leads to the creation of extremely stable complexes, particularly with transition and alkaline earth metals. It’s perfect for:
- Heavy metal ion removal from solutions
- Stabilizing metal ions in formulations
- Enhancing shelf life of personal care and food products
- Water softening in industrial detergents
Stability Constants (Log K):
Ca²⁺: ~10.7
Fe³⁺: ~25.1
Cu²⁺: ~18.8
HEDP: Moderate Chelator, Excellent Inhibitor
HEDP, with its phosphonic acid groups, also binds metal ions but with lower stability constants than EDTA. However, it excels at:
- Threshold inhibition: preventing crystal growth of scales like calcium carbonate, calcium sulfate, and barium sulfate
- Corrosion inhibition: forming a protective film on metal surfaces (especially steel)
It also retards precipitation rather than removing metal ions from the solution entirely.

pH Stability and Thermal Resistance
EDTA
- Stable in neutral and mildly alkaline environments
- Loses efficiency in strongly acidic or strongly alkaline pH
- Not recommended for high-temperature applications
HEDP
Chemically stable in wide pH ranges (2–12)
Because it is resistant to hydrolysis at high temperatures, it can be used for:
- Cooling water systems
- Oilfield water injection
- Boiler treatment
This thermal and pH resilience is one of the biggest differentiators between EDTA and HEDP.
Biodegradability and Environmental Profile
EDTA: Persistent Pollutant
Poor biodegradability under standard wastewater treatment
Can mobilize heavy metals in soil and water, increasing bioavailability and toxicity
Environmental regulations are increasingly limiting EDTA discharge
HEDP: Moderately Biodegradable
Not readily biodegradable, but degrades slowly via photolysis and chemical oxidation
Less hazardous in aquatic environments compared to EDTA
Does not enhance mobility of heavy metals to the same degree
HEDP has a better environmental profile, particularly in wastewater-heavy applications like cooling systems or textile effluents.
Industrial Applications: EDTA vs. HEDP
| Industry | EDTA | HEDP |
| Cosmetics | Preservative booster, sequesters metal ions | Rarely used |
| Pharmaceuticals | Chelates trace metals in formulations | Rarely used |
| Water Treatment | Water softening, metal ion control | Scale and corrosion inhibitor |
| Detergents | Enhances cleaning by removing Ca/Mg | Stabilizes bleach, prevents scale |
| Oil & Gas | Not stable at high temps/pressure | Used in scale control in pipelines |
| Textiles | Removes metal impurities before dyeing | Prevents scale on machinery |
| Food Processing | Limited use (E385 – disodium EDTA) | Not allowed in food products |
| Pulp & Paper | Brightening agent stabilizer | Boiler scale control |
Compatibility with Oxidizers and Formulations
EDTA
- Incompatible with strong oxidizing agents (e.g., sodium hypochlorite), as it may decompose or form undesired byproducts
- Most surfactants and preservatives included in personal care products are compatible with
HEDP
- Compatible with sodium hypochlorite and peroxides
- Stabilizes chlorine-based cleaners and disinfectants
- Prevents precipitation of Ca/Mg in bleach systems
Common in industrial sanitizers and bleach-based cleaners
Salt Forms and Solubility
EDTA Forms
Acid form (poorly soluble)
- Disodium EDTA (EDTA-2Na): most common in cosmetics
- Tetrasodium EDTA (EDTA-4Na): highly water-soluble, alkaline pH
- Solubility: ~100g/L (EDTA-2Na in water at 25°C)
HEDP Forms
HEDP acid (low pH)
- HEDP-Na (1, 2, 4 sodium salt forms) for better solubility and pH control
- Used in liquid formulations or blends with polycarboxylates
- Solubility: Varies with salt form; generally high
Safety and Handling
EDTA
- Low acute toxicity
- Can irritate eyes and skin in concentrated form
- Handle with PPE in industrial settings
HEDP
- Also low toxicity
- Skin and mucous membranes can become irritated by strong acids.
- Avoid mixing with concentrated alkalis without pH control
Both require standard chemical handling protocols including gloves, eye protection, and proper storage.
Cost and Supply Chain Consideration
- EDTA is generally more expensive than HEDP due to purification, regulatory compliance, and market demand in cosmetics/pharmaceuticals.
- HEDP is more economical in large-scale industrial processes like cooling systems or oilfields.
As a chemical raw material supplier, we offer bulk pricing, technical documentation (MSDS, COA), and custom formulations depending on your application.
While EDTA and HEDP share some overlap in chelation and scale control, they are not interchangeable.
Choose EDTA for cosmetic, pharmaceutical, and specialty formulations that demand high-purity metal sequestration.
Opt for HEDP in industrial applications, especially where high temperature, pH tolerance, and scale inhibition are critical.
As your trusted chemical raw material supplier, we provide technical support and tailored solutions to meet your exact formulation needs—whether you’re stabilizing a cream or preventing scale in a power plant.