In homes, hospitals, and laboratories, alcohol-based disinfectants are essential tools for hygiene and sanitation. There are significant differences between the two in terms of composition, strength, applications, and safety, despite the fact that they are occasionally confused. Knowing these distinctions can assist manufacturers, healthcare professionals, and consumers in selecting the best product for their needs.

What Is Isopropyl Alcohol?
2-propanol, another name for isopropyl alcohol (IPA), is a colorless, flammable chemical molecule that has a distinct smell. Its molecular formula is C₃H₈O (or CH₃CHOHCH₃), and it belongs to the alcohol family of organic compounds.
Key features of isopropyl alcohol:
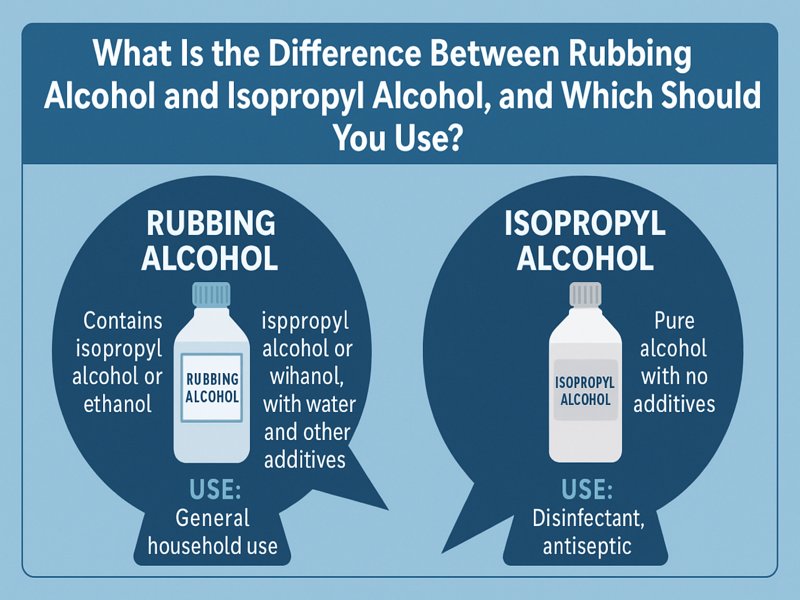
- Pure compound (usually 70%, 91%, or 99% concentrations in solutions)
- Quick-evaporating
- Used as a solvent and disinfectant
- Antimicrobial and antiseptic
Common uses:
- Medical sterilization
- Electronics cleaning
- Surface disinfection
- Cosmetic formulations
- Solvent in paints, inks, and resins
When referring to isopropyl alcohol, people typically mean a solution that is pure IPA diluted with water to various concentrations. These concentrations impact its disinfectant properties, which we’ll explore later.

What Is Rubbing Alcohol?
An antiseptic solution comprising isopropyl alcohol or ethanol as the active ingredient, water, and additional additives such as denaturants, perfumes, or coloring compounds is referred to as “rubbing alcohol.”
In the U.S., rubbing alcohol generally refers to:
- 70% isopropyl alcohol with additives OR
- 70% ethyl alcohol with additives
Key differences in rubbing alcohol:
- Contains additives (e.g., bitterants, denaturants)
- Made for topical use only
- Slightly less pure than pure isopropyl alcohol
- Less expensive, commonly used in households
Because of its additives, rubbing alcohol is not suitable for internal use or ingestion and is designed strictly for external application.
Important Distinctions Between Rubbing Alcohol and Isopropyl Alcohol
| Feature | Isopropyl Alcohol | Rubbing Alcohol |
| Main Ingredient | Isopropyl alcohol | Isopropyl or ethyl alcohol |
| Purity | Highly pure (up to 99%) | Mixed with water and additives |
| Additives | Usually none (unless for specific uses) | Yes (fragrance, bittering agents, denaturants) |
| Concentration Range | 60% to 99% | Typically 70% |
| Common Use | Industrial, lab, and medical cleaning | Household, first-aid |
| Evaporation Speed | Faster (especially 91–99%) | Slightly slower due to water and additives |
| Toxicity if Ingested | High | Higher due to added denaturants |
| Price | Slightly more expensive | Generally cheaper |
Effectiveness as a Disinfectant
Rubbing alcohol and isopropyl alcohol both work well as disinfectants, but concentration is key.
70% Isopropyl Alcohol: This is considered the optimal concentration for killing bacteria, viruses, and fungi. The presence of 30% water slows down evaporation, allowing alcohol to penetrate cell membranes and denature proteins more effectively.
91% or 99% Isopropyl Alcohol: While stronger, these concentrations evaporate quickly, which may not allow enough contact time to fully disinfect. However, they are ideal for electronics and cleaning moisture-sensitive surfaces.
Rubbing Alcohol (70%): Since most rubbing alcohol is 70% IPA with added ingredients, it works similarly to 70% pure IPA. However, the additives may interfere with sensitive surfaces like electronics or lenses.
In conclusion, 70% isopropyl alcohol or rubbing alcohol is excellent for sanitizing skin and surfaces. For cleaning circuit boards or lenses, higher-purity isopropyl alcohol (91%–99%) is preferred.
Uses in Different Applications
Let’s break down where each type of alcohol shines best:
a. Medical and First Aid
- Rubbing alcohol is designed for topical use to disinfect cuts, wounds, and skin before injections.
- Isopropyl alcohol (especially 70%) is used in hospitals for sterilizing equipment, cleaning surgical tools, and preventing infections.
b. Household Cleaning
- Rubbing alcohol is ideal for cleaning countertops, mirrors, phones, and bathrooms.
- Isopropyl alcohol at 70% or above is better suited for heavy-duty cleaning or preparing surfaces before painting or sealing.
c. Industrial and Laboratory
- 99% Isopropyl alcohol is used for cleaning optical devices, removing adhesives, and degreasing machinery.
- Because of the possibility of additive contamination, rubbing alcohol is often not used in laboratories.
d. Cosmetics and Skincare
- Isopropyl alcohol is used in toners, aftershaves, and acne treatments in controlled concentrations.
- Rubbing alcohol is not typically used in cosmetic production due to its impurities.
Safety and Handling
Rubbing alcohol and isopropyl alcohol are both combustible and therefore to be handled carefully.
a. Health Risks
- Skin contact: With extended usage, both may result in dryness or irritation.
- Inhalation: High exposure can lead to dizziness or respiratory irritation.
- Ingestion: Extremely dangerous, especially rubbing alcohol, due to toxic additives.
b. Storage
- Keep out of direct heat or flame and in a cool, dry location.
- Containers should be kept firmly sealed to avoid evaporation.
- Keep away from food and beverages.
c. Environmental Impact
- Isopropyl alcohol is readily biodegradable, but large spills can harm aquatic ecosystems.
- Avoid pouring either substance down drains in large quantities.
Myths and Misconceptions
Myth #1: Higher concentration = better disinfection
Truth: 70% isopropyl alcohol is more effective for disinfection than 99% due to slower evaporation and better cell wall penetration.
Myth #2: Rubbing alcohol is just a cheaper version of IPA
Truth: Rubbing alcohol may contain ingredients not suitable for sensitive surfaces or lab use.
Myth #3: It’s safe to use rubbing alcohol on all electronics
Truth: Additives in rubbing alcohol may leave residues. Always use 99% IPA for electronics.
Which Should You Use?
| Use Case | Recommended Alcohol |
| Sterilizing medical tools | 70% Isopropyl alcohol |
| Cleaning electronics | 91%–99% Isopropyl alcohol |
| Treating minor wounds | Rubbing alcohol (70%) |
| Household cleaning | Rubbing alcohol or 70% IPA |
| Cosmetic formulations | High-purity isopropyl alcohol |
| DIY hand sanitizer | 99% IPA diluted to 70% with aloe |
Regulatory Standards
In the U.S., both products are regulated:
- Isopropyl Alcohol is classified by the FDA when used in OTC drug products and by the EPA when used in disinfectants.
- Rubbing Alcohol must meet USP (United States Pharmacopeia) standards and typically contains bittering agents to prevent ingestion.
Always check for USP grade, pharmaceutical grade, or technical grade, depending on your application.
Know What You’re Using
Although isopropyl alcohol and rubbing alcohol are closely related, they’re not interchangeable in every scenario. The purity, additives, and intended uses make a difference, especially when dealing with electronics, cosmetics, or critical sanitation.
- Choose isopropyl alcohol (70%) for medical-grade disinfection and skin applications.
- Choose isopropyl alcohol (91%–99%) for delicate surfaces, electronics, and solvent use.
- Choose rubbing alcohol for general home cleaning and first-aid kits, especially when cost and availability are considerations.